Vaccination strategy in liver transplantation – are we keeping up with global trends?
Zuzana Podmanická1, Světlana Adamcová Selčanová2, Eliška Lovratová3, Zuzana Krištúfková4, Ľubomír Skladaný5
+ Affiliation
Summary
Patients with advanced chronic liver disease (ACLD), those awaiting liver transplantation (LT), and post-transplant recipients face a high risk of infectious complications due to immune dysfunction. Vaccination is a key preventive strategy to protect this high-risk group from severe infections. The effectiveness of vaccines depends on the timing of administration – optimal vaccination occurs in the early stages of liver disease before transplantation when the immune response is strongest. Post-transplant vaccination is possible, but requires stabilization of immunosuppressive therapy. The administration of live vaccines after LT remains controversial, although recent research suggests their potential safety in specific cases. This paper summarizes recommended vaccination strategies in Europe and worldwide, evaluates the effectiveness of vaccination in this patient population, and highlights the need for further research to optimize immunization protocols for patients with chronic liver diseases and transplant recipients.
Keywords
advanced chronic liver disease, liver transplantation, vaccination, immune dysfunction, infectious complications, immunosuppressive therapy
Introduction
Advanced chronic liver disease (ACLD) is a condition frequently if not universally associated with the development of systemic inflammation and immune dysregulation, known as the cirrhosis--associated immune dysfunction syndrome (CAID) [1,2]. Development of this secondary immunodeficiency leads to, among other consequences, reduced immune defense against infections [2–5]. One of the preventive measures to protect high-risk patients from severe infections is vaccination. As many ACLD patients progress to end-stage liver disease (ESLD) or acute-on-chronic liver failure (ACLF), requiring consideration for liver transplantation (LT), they become particularly vulnerable [6–9]. Therefore, maximal efforts should be made to ensure they receive preventive vaccination [9–11].
According to available data, pre-LT vaccination should be administered at a time before LT when the patient still has the potential to develop sufficient immunity [12]. If vaccination is delayed until advanced disease stages, it becomes less effective or the full vaccination schedule may not be completed [13]. Vaccination can also be administered after LT, but this period carries many challenges which are subject of ongoing research. Current guidelines provide a good framework for management of patients with ACLD both before and after LT [13–15].
This paper aims to summarize the recommended vaccination strategies for these patient groups.
Recommended general vaccination strategies for high-risk groups in Europe and outside Europe
The fundamental principles of vaccination for both pediatric and adult populations, including high-risk groups, are summarized in the Centers for Disease Control and Prevention (CDC) guidelines, which are annually updated and published in collaboration with Advisory Committee on Immunization Practices (ACIP) in the United States [15–18]. Among the high-risk groups, patients with ACLD are also included [13,14].
The CDC recommendations classify patients with chronic liver diseases into two groups:
1. patients with chronic hepatitis [19,20];
2. patients with ACLD who are further categorized into:
- a) patients with portal hypertension;
- b) patients with functional asplenia;
- c) patients with immunodeficiency (CAID) [1,2,21,22].
Each group is assigned an appropriate vaccination schedule. Patients are recommended to receive vaccinations against 15 infectious diseases, including Influenza, Tetanus, Diphtheria, Pertussis, Measles, Mumps, Rubella, Varicella, Human papillomavirus (HPV), Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae, Hepatitis A virus, and Hepatitis B virus. Vaccination is most effective when administered in the early stages of the disease (Tab. 1) [15].
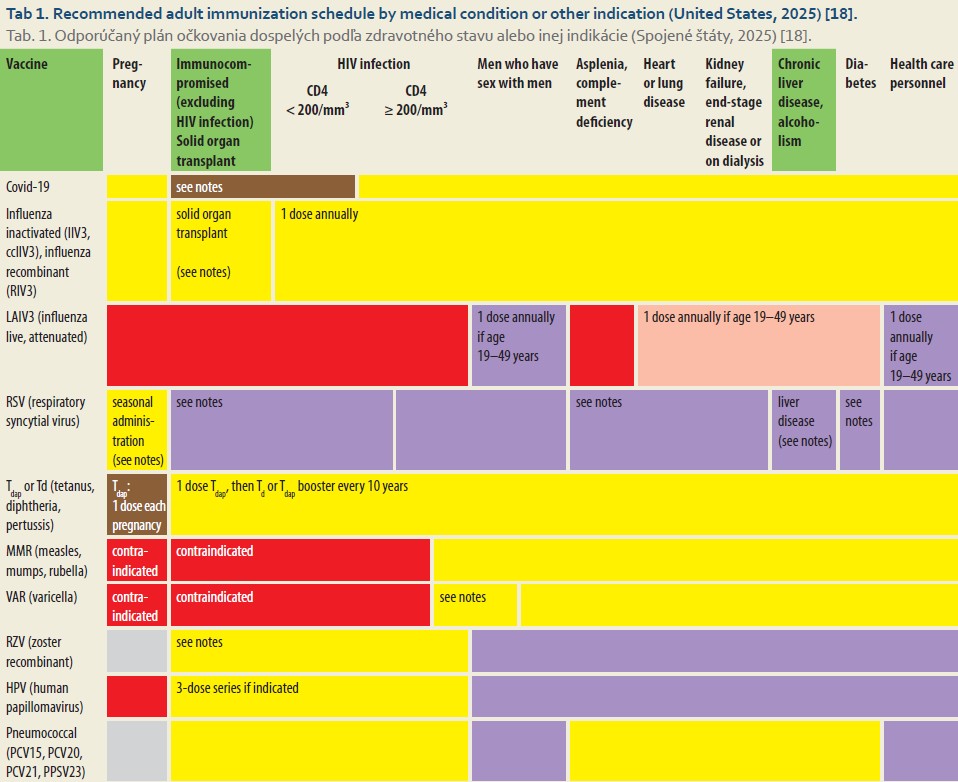
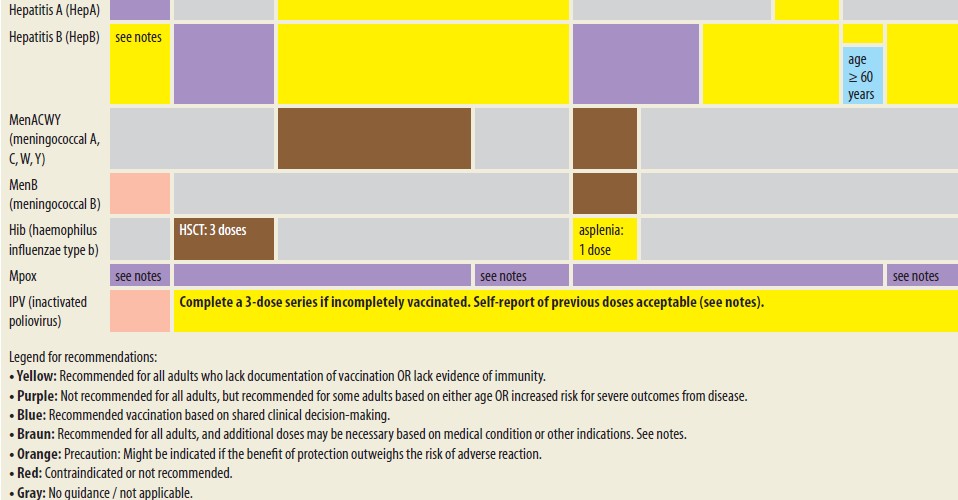
Basic principles and timing of vaccination
The proper timing of vaccination in high--risk groups, specifically in the pre-transplantation and post-transplantation periods, has been a long-discussed topic. It has been proven that vaccination with inactivated (non-live) vaccines before transplantation is safe; however, in advanced stages of ACLD, it may be less effective [13,23]. In the case of live vaccines, it is recommended to maintain an interval of at least 4 weeks before LT [13].
After LT, recommendations for vaccination with inactivated vaccines vary among different authors, mainly regarding timing (2, 3, or 6 months after LT) [11,24,25]. The fundamental principle is that vaccination should take place when the dose of immunosuppressive therapy is stabilized and no longer in the induction phase [11,24,25]. In contrast to inactivated vaccines, vaccination with live (attenuated) vaccines has traditionally not been recommended in the adult population after LT [11,13]. However, recent data on efficacy and safety suggest that their use after solid organ transplantation (SOT) could be a viable strategy (Tab. 2) [25–31].
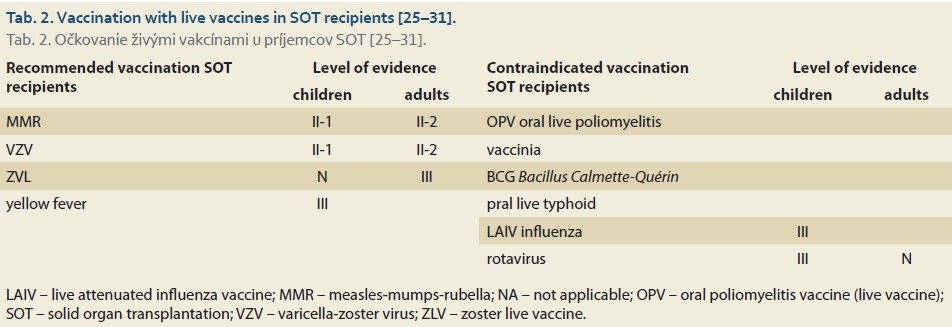
The primary consideration of administering live vaccines to severely immunocompromised individuals is the risk of dissemination of the vaccine strain and ensuing graft rejection [12,13,29]. Therefore, when considering their application, it is crucial to take into account the immunological profile of the patients, particularly the absolute count of CD4+ T-helper lymphocytes and the risk of infection with the so-called “wild” strain [12,30,32–36]. Vaccination with live vaccines is contraindicated in adult patients when CD4+ T-helper lymphocytes are below 15% (< 200 × 10⁶/L). In patients undergoing immunosuppressive therapy, an absolute CD4+ lymphocyte count of > 400 × 10⁶/L is recommended [30]. Besides the count and composition of lymphocytes, administration of live vaccines also depends on the dosage of immunosuppressive therapy, which is arbitrarily determined based on expert consensus [33]. However, contraindications and safe vaccination protocols are not yet sufficiently supported by literature for all immunosuppressive and biological therapies used in LT (Tab. 3) [33,37–39].
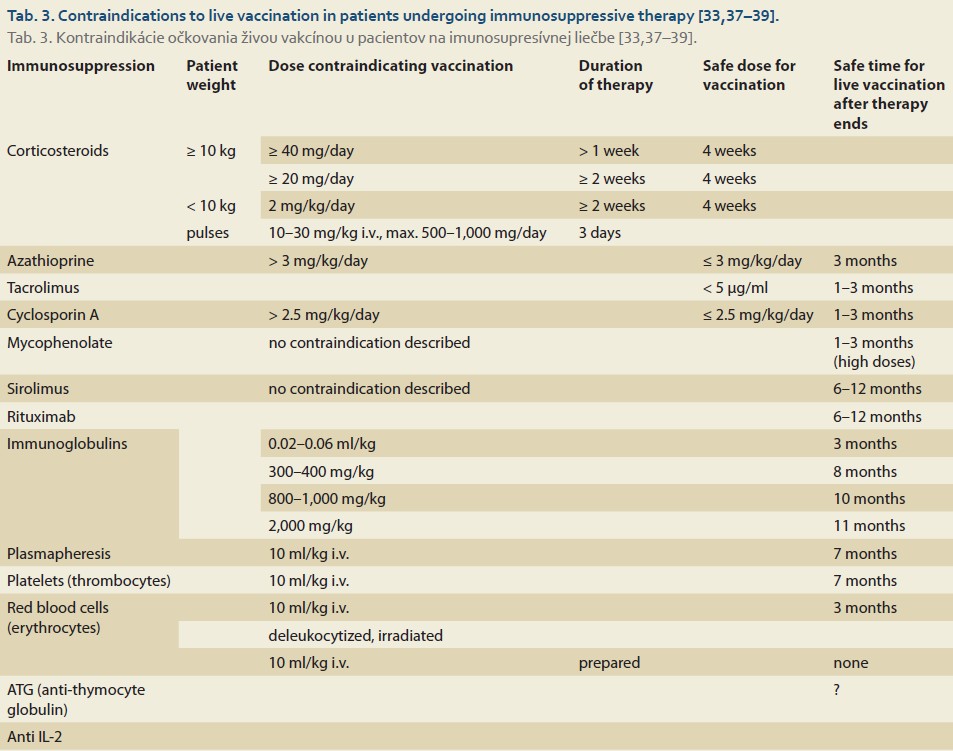
Given the above considerations, the preferred approach is to administer live attenuated vaccines at least 2–4 weeks before LT [15,30,33]. In cases where vaccination is contraindicated, risk assessment of an unvaccinated individual (patient) is recommended, and if at high-risk, focusing on a vaccinated milieu (e. g., family) is considered. This involves vaccinating close contacts (household members, healthcare personnel) following the so-called “cocoon” strategy [40].
Effectiveness of vaccination in a liver transplantation setting
The effectiveness and safety of vaccination depend on many factors. It is usually assessed based on the development of a protective antibody titer within 2–4 weeks post-vaccination [41]. However, this remains an area of ongoing research in high-risk patients with varying degrees of immune system impairment and those receiving immunosuppressive therapy [13,32]. One key factor influencing vaccination effectiveness is the timing of vaccination. The highest efficacy is observed in the early stages of chronic liver disease [14,15,18], while effectiveness decreases as liver damage progresses [2,14]. According to available studies, mild (non-protective) seroconversion is not significantly affected by gender and body mass index (BMI); of note, there are no differences between decompensated patients classified as Child-Pugh B and Child-Pugh C [42]. Studies from transplantation centers report a higher effectiveness of vaccination before LT [13]. However, successful immunization can still be achieved after LT [29]. Over the past 30+ years, numerous clinical studies have been published on patients after SOT. However, these studies are highly heterogeneous and predominantly focused on pediatric patients [41]. Findings indicate that while SOT patients are capable of mounting an immune response, their response to most vaccines is lower compared to the healthy population. The extent of this impaired immune response varies depending on the type of vaccine, transplanted organ, and patient’s age [43]. An overview of immune responses to certain non-live vaccines after LT is displayed in Tab. 4 [41].
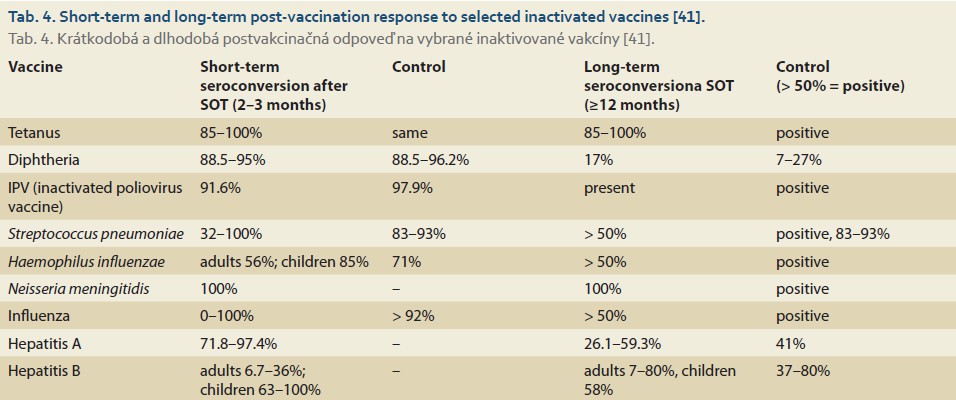
We present a summary of vaccination effectiveness after live vaccines following SOT in the form of a review of available interventional studies, again predominantly based on the pediatric population (Tab. 5).
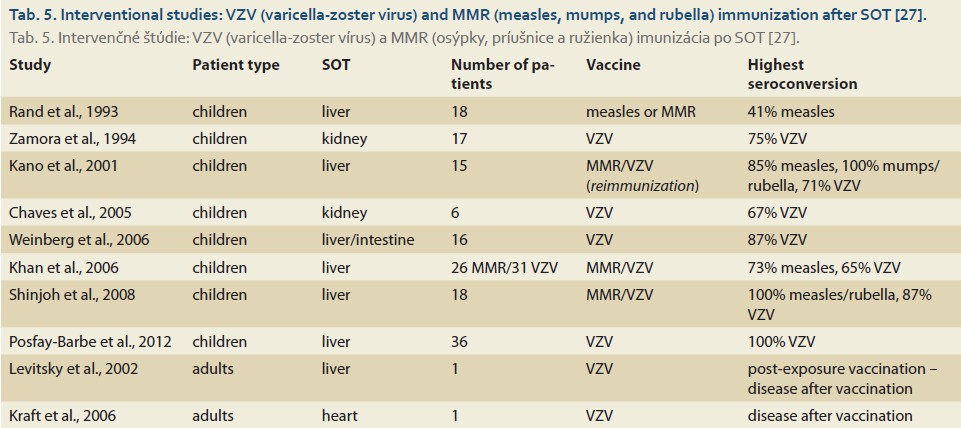
Protective antibody titers after vaccination
Most transplant centers monitor the effectiveness of vaccination through routine serological testing. In cases of insufficient serological response (absence of specific IgG antibodies in peripheral blood), revaccination – the administration of a booster dose – is indicated in most cases [41]. Available literature provides protective antibody titer data for 26 vaccines (Tab. 6) [42].
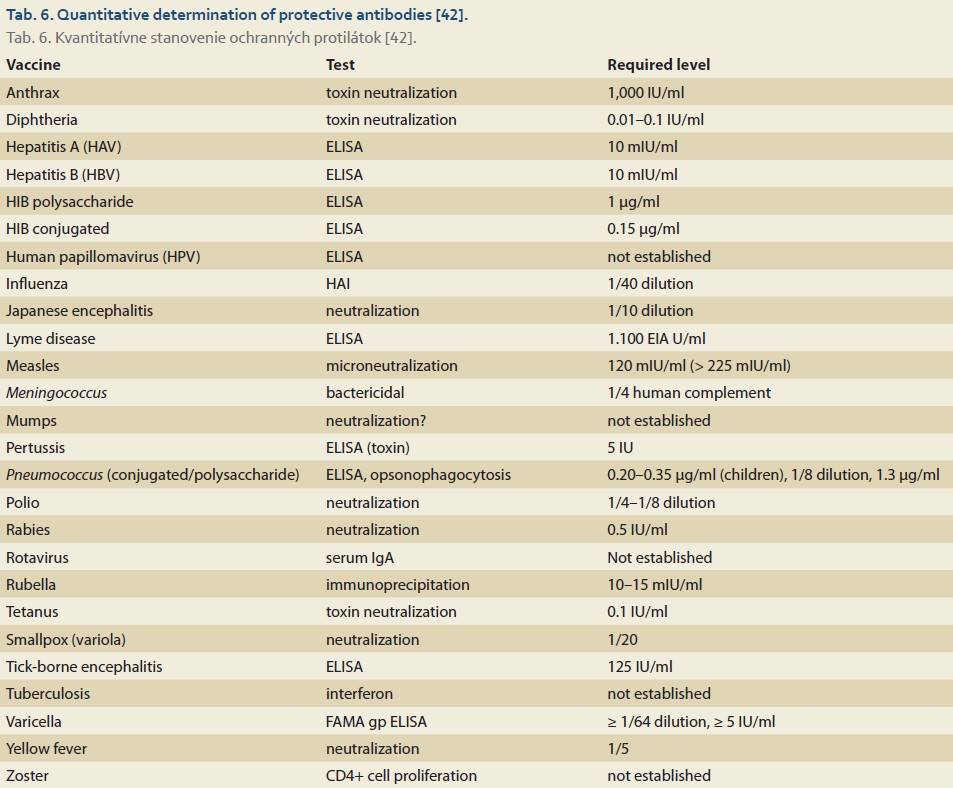
In the context of LT, multiple studies have focused on the effectiveness of Hepatitis B virus (HBV) vaccination, primarily due to the risk of developing de novo acquired hepatitis B, which may lead to graft failure [43,44]. In the general population, an anti-HBs titer > 10 mIU/ml is considered protective. However, in patients with advanced chronic liver disease (ACLD) and post--LT, authors suggest that a seroprotective titer should be defined as anti-HBs > 100 mIU/ml [43,44].
Revaccination – “booster” doses before solid organ transplantation
For patients awaiting SOT, it is optimal to complete all planned vaccinations well in advance of surgery to allow for the administration of the full vaccination protocol. If low peripheral levels of specific antibodies are detected before SOT, most transplant centers recommend revaccination (booster dose) [45]. According to available literature, in patients under 6 years of age, a booster dose may be administered at a minimum interval of 12 months after primary vaccination. In patients over 7 years and in adults with completed primary vaccination, booster doses are given as needed, such as reduced diphtheria toxoid vaccine (dTap), which should be administered at least 2 weeks before the planned transplantation [32]. For post-SOT patients, annual influenza vaccination is recommended, a 23-valent pneumococcal polysaccharide vaccine should be boosted every 2–5 years, a tetanus toxoid booster every 5 years, and a diphtheria toxoid booster every 10 years [13]. Circulating specific IgG antibodies following protein-based vaccines can be detected 3–4 weeks post-vaccination. A fourfold increase in antibody titers is considered a sufficient post-vaccination response. Although specific post-vaccination antibody levels in peripheral blood may decline, this does not indicate a lack of immunization. Upon re-exposure to the antigen, tissue memory cells (T and B lymphocytes) become reactivated, leading to a rapid antibody response [46–48]. Memory B and T cells can persist for several decades [13,49,50]. However, in the context of SOT, the lifespan of memory T lymphocytes has not yet been fully determined, nor is the effect of immunosuppressive therapy on immune memory cells completely understood [13,46,50].
Vaccination of close contacts
According to U.S. guidelines, vaccination of the SOT candidate alone is insufficient, and immunization of close contacts is also recommended [51]. Healthcare workers, especially caregivers, close contacts, and family members, should be fully immunized according to national vaccination schedules [30,51–54]. Healthy, immunocompetent individuals can receive both inactivated and live vaccines (Tab. 7) [55].

Vaccination of close contacts can prevent the transplanted patient from exposure to wild-type viruses [51].
Conclusion
Patients with advanced chronic liver disease (ACLD), those awaiting LT, and post-LT recipients face a high risk of infectious complications, which significantly contribute to increased morbidity and mortality. One of the fundamental preventive measures to protect these high-risk patients is vaccination, not only for the patients themselves but also for their close contacts. To date, standardized global criteria for vaccination before and after LT have not been established. According to available literature, timing is one of the key factors influencing vaccine effectiveness. The optimal period for vaccination appears to be the earliest-captured stage of liver disease before LT. In the post-LT period, the fundamental principle of vaccination is ensuring a stable immunosuppressive regimen. However, the use of live vaccines after LT remains the area of further research. A deeper investigation into this cohort of patients will allow the development of evidence-based recommendations, ensuring effective immunization and optimal protection for transplant recipients.
ORCID authors
Z. Podmanická 0000-0001-5683-3261,
S. Adamcová Selčanová 0000-0001-8181-1937,
Z. Krištúfková 0000-0003-1728-9659,
Ľ. Skladaný 0000-0001-5171-3623.
Submitted/Doručené: 25. 3. 2025
Accepted/Prijaté: 2. 4. 2025
Corresponding author
Svetlana Adamcová Selčanová, MD, PhD.
2nd Department of Internal Medicine
Div. HEGITO (Hepatology, Gastroenterology, and Transplantation Department)
Slovak Medical University Faculty of Medicine, Roosevelt University Hospital
Nám. L. Svobodu 1
975 17 Banská Bystrica
sselcanova@gmail.com
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3): 743–752. doi: 10.1016/j.jhep.2015.05.022.
2. Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014; 60(6): 1385–1396. doi: 10.1016/j.jhep.2014.01.022.
3. Bonnel AR, Bunchorntavakul C, Rajender Reddy K. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2011; 9(9): 727–738. doi: 10.1016/j.cgh.2011.02.031.
4. Noor MT, Manoria P. Immune dysfunction in cirrhosis. J Clin Transl Hepatol 2017; 5(1): 50–58. doi: 10.14218/JCTH.2016.00056.
5. Irvine KM, Ratnasekera I, Powell EE et al. Causes and consequences of innate immune dysfunction in cirrhosis. Front Immunol 2019; 10: 293. doi: 10.3389/fimmu.2019.00293.
6. Jalan R, Gines P, Olson JC et al. Acute-on-chronic liver failure. J Hepatol 2012; 57(6): 1336–1348. doi: 10.1016/j.jhep.2012.06.026.
7. Price J. Referral for liver transplantation. 2024 [online]. Available from: https: //www.hepatitisc.uw.edu/go/management-cirrhosis-related-complications/liver-transplantation-referral/ core-concept/all.
8. Kim WR, Lake JR, Smith JM et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(Suppl 1): 172–253. doi: 10.1111/ajt.14998.
9. Kotton CN, Hibberd PL. Immunizations in solid organ transplant candidates and recipients. 2025 [online]. Available from: https: //www.uptodate.com/contents/immunizations-in-solid-organ-transplant-candidates-and-recipients?search=Immunizations%20in%20solid%20organ% 20transplant%20candidates%20and%20reci- pients&source=search_result&selectedTitle=1% 7E150&usage_type=default&display_rank=1.
10. Hibberd PL, Rubin HR. Approach to immunization in the immunosuppressed host. Infect Dis Clin North Am 1990; 4(1): 123–142. doi: 10.1016/S0891-5520(18)30332-2.
11. Danziger-Isakov L, Kumar D. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4): 311. doi: 10.1111/ajt.12190.
12. Rubin LG, Levin MJ, Ljungman P et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58(3): e44–e100. doi: 10.1093/cid/cit816.
13. Duchini A, Goss JA, Karpen S et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev 2003; 16(3): 357–364. doi: 10.1128/CMR.16.3.357-364.2003.
14. Kim DK, Riley LE, Hunter P et al. Recommended immunization schedule for adults aged 19 years or older, United States, 2018. Ann Intern Med 2018; 168(3): 210–220. doi: 10.7326/M17-3439.
15. Kim DK, Hunter P. Recommended adult immunization schedule for ages 19 years or older. United States 2019. Ann Intern Med 2019; 170(3): 182–192. doi: 10.7326/M18-3603.
16. Krištúfková Z. Základy vakcinológie. Banská Bystrica: PRO 2022.
17. CDC. Recommended child and adolescent immunization schedule for ages 18 years or younger. 2019 [online]. Available from: chrome-extension: //efaidnbmnnnibpcajpcglclefindm kaj/https: //www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-combined-schedule-bw.pdf.
18. CDC. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States. 2025 [online]. Dostupné z: https: //www.cdc.gov/vaccines/schedules/.
19. Dienstag JL. Chronic hepatitis. In: Kasper D, Fauci A, Hauser S et al. (eds). Harrison‘s principles of internal medicine. New York: McGraw-Hill 2015.
20. Munjal YP, Sharma SK. Textbook of medicine. New Delhi: JP Medical Ltd. 2012.
21. Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 2004; 39(2): 280–282. doi: 10.1002/hep.20068.
22. Kikineska L, Perifanis V, Vasiliadis T. Functional hyposplenism. Hippokratia 2014; 18(1): 7–11.
23. Rožnovský L, Orságová I, Petroušová L et al. Low response rate to a vaccination against hepatitis B in patients with end-stage liver disease. Klin Mikrobiol Infekc Lek 2010; 5(16): 179–181.
24. Karuthu S, Blumberg EA. Common infections in kidney transplant recipients. Clin J Am Soc Nephrol 2012; 7(12): 2058–2070. doi: 10.2215/CJN.04410512.
25. Danziger-Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant 2019; 33(4): e13563. doi: 10.1111/ctr.13563.
26. Danerseau AM, Robinson JL. Efficacy and safety of measles, mumps, rubella, and varicella live viral vaccines in transplant recipients receiving immunosuppressive drugs. World J Pediatr 2008; 4(4): 254–258. doi: 10.1007/s12519-008-0048-4.
27. L’Huillier AG, Kumar D. Immunizations in solid organ and hematopoietic stem cell transplant patients: a comprehensive review. Hum Vaccin Immunother 2015; 11(12): 2852–2863. doi: 10.1080/21645515.2015.1070934.
28. Pittet LF, Posfay-Barbe KM. Immunization in transplantation: a review of the recent literature. Curr Opin Organ Transplant 2013; 18(5): 543–548. doi: 10.1097/MOT.0b013e3283636c88.
29. Kraft JN, Shaw JC. Varicella infection caused by Oka strain vaccine in a heart transplant recipient. Arch Dermatol 2006; 142(7): 943–945. doi: 10.1001/archderm.142.7.943.
30. L’Huillier AG, Posfay-Barbe KM. Live viral vaccines in immunocompromised patients. Future Virol 2014; 9(2): 161–171. doi: 10.2217/fvl.13.141.10.1097/MOT.0b013e32836 41e75.
31. Croce E, Hatz C, Jonker EF et al. Safety of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation, or after bone-marrow transplantation – a systematic review of randomized trials, observational studies, and case reports. Vaccine 2017; 35(9): 1216–1226. doi: 10.1016/j.vaccine.2016.12.020.
32. Jeseňák M, Urbančíková I. Očkovanie v špeciálnych situáciách. Praha: Mladá fronta 2019.
33. Health Service Executive. Chapter 3: Immunisation of immunocompromised persons. 2019 [online]. Dostupné z: https: //www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter3.pdf.
34. Bonilla FA. Update: vaccines in primary immunodeficiency. J Allergy Clin Immunol 2018; 141(2): 474–481. doi: 10.1016/j.jaci.2017.12.968.
35. Kimberlin DW. Red book 2018–2021: report of the Committee on Infectious Diseases. Itasca: American Academy of Pediatrics 2018.
36. Levin MJ, Gershon AA, Weinberg A et al. Administration of live varicella vaccine to HIV-infected children with current or past significant depression of CD4 (+) T cells. J Infect Dis 2006; 194(2): 247–255. doi: 10.1086/505430.
37. Duca P, Del Pont JM, D’Agostino D. Successful immune response to recombinant hepatitis B vaccine in children after liver transplantation. J Pediatr Gastroenterol Nutr 2001; 32(2): 168–170. doi: 10.1097/00005176-200102000-00012.
38. National Center for Immunization and Respiratory Diseases. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60(2): 1–64.
39. Kroger AT, Strikas RA. General recommendations for vaccination & immunoprophylaxis, CDC, Chapter 2. 2017 [online]. Dostupné z: https: //wwwnc.cdc.gov/travel/yellowbook/ 2018/the-pre-travel-consultation/general-recommendations-for-vaccination-immuno- prophylaxis.
40. Nitsch-Osuch A. Cocoon strategy of vaccinations: benefits and limitations. 2017 [online]. Available from: https: //www.intechopen.com/chapters/55384.
41. Eckerle I, Rosenberger KD, Zwahlen M et al. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One 2013; 8(2): e56974. doi: 10.1371/journal.pone.0056974.
42. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17(7): 1055–1065. doi: 10.1128/CVI.00131-10.
43. Domínguez M, Bárcena R, López-Sanroman A et al. Vaccination against hepatitis B virus in cirrhotic patients on the liver transplant waiting list. Liver Transpl 2000; 6(4): 440–442. doi: 10.1053/jlts.2000.8596.
44. Villeneuve E, Vincelette J, Villeneuve JP. Ineffectiveness of hepatitis B vaccination in cirrhotic patients waiting for liver transplantation. Can J Gastroenterol 2000; 14(Suppl B): 59B–62B. doi: 10.1155/2000/548206.
45. Berger C. Recommendations for immunization of solid organ transplant (SOT) candidates and recipients. 2014 [online]. Available from: https: //www.bag.admin.ch/dam/bag/fr/dokumente/mt/i-und-b/ekif/background-immunization-sot.pdf.download.pdf/background-immunization-sot-fr.pdf.
46. Sarkander J, Hojyo S, Tokoyoda K. Vaccination to gain humoral immune memory. Clin Transl Immunol 2016; 5(12): e120. doi: 10.1038/cti.2016.83.
47. Ciabattini A, Pettini E, Medaglini D. CD4 (+) T cell priming as a biomarker to study immune response to preventive vaccines. Front Immunol 2013; 4: 421. doi: 10.3389/fimmu.2013.00421.
48. Cruse JM, Lewis RE. Atlas of immunology. Boca Raton: CRC Press/Taylor & Francis 2010.
49. Lefevre EA, Carr BV, Prentice H et al. A quantitative assessment of primary and secondary immune responses in cattle using a B cell ELISPOT assay. Vet Res 2009; 40(1): 3. doi: 10.1051/vetres: 2008053.
50. Manz RA, Hauser AE, Hiepe F et al. Maintenance of serum antibody levels. Annu Rev Immunol 2005; 23: 367–386. doi: 10.1146/annurev.immunol.23.021704.115723.
51. Danzinger-Isakov L, Kumar D. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant 2009; 9(Suppl 4): S258–S262. doi: 10.1111/j.1600-6143.2009.02915.x.
52. Liang JL, Tiwari T, Moro P et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018; 67(2): 1–44. doi: 10.15585/mmwr.rr6702a1.
53. Ljugman P, Engelhard D, de la Cámara R et al. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant 2005; 35(8): 737–746. doi: 10.1038/sj.bmt.1704861.
54. Tomblyn M, Chiller T, Einsele H et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15(10): 1143–1238. doi: 10.1016/j.bbmt.2009.06.019.
55. Armstrong C. IDSA releases recommendations on vaccinations in immunocompromised patients. Am Fam Physician 2014; 90(9): 664–666.