From compensation to decompensation: dynamics, risk factors, and management of cirrhosis
Daniela Žilinčanová1, Světlana Adamcová Selčanová1, Daniel Ján Havaj1, Jana Čiefová1, Karolína Šulejová1, Michal Žilinčan2, Teodor Takáč1, Ľubomír Skladaný1
+ Affiliation
Summary
Cirrhosis represents the final stage of advanced chronic liver disease (ACLD) with the transition from compensated to decompensated stages marking a critical point in patient prognosis. This review explores the dynamics, risk factors, and management of both acute (AD) and non--acute decompensation (NAD) in cirrhosis. It highlights key pathophysiological mechanisms such as portal hypertension, systemic inflammation, and bacterial translocation, and classifies clinical phenotypes based on recent findings from the landmark CANONIC and PREDICT studies. The article aimed at underscoring importance of early diagnosis, standardized targeting of the key pathophysiological domains of portal hypertension, individualized management strategies, and emerging approaches to achieve recompensation.
Keywords
decompensation, portal hypertension, systematic inflammation, acute-on-chronic liver failure (ACLF), recompensation
Introduction
Cirrhosis, as the terminal stage of advanced chronic liver disease (ACLD), represents a critical global health challenge. Its prevalence and impact vary by geographic region, yet it remains a significant contributor to global mortality and morbidity. Liver diseases, including cirrhosis, account for approximately 2 million deaths annually, with nearly half of these attributed to cirrhosis-related complications [1,2]. The condition is characterized by progressive hepatic parenchymal damage leading to portal hypertension, systemic inflammation, and organ dysfunction [3]. The transition from compensated (cACLD) to decompensated cirrhosis (dACLD) is a pivotal event in the disease course, as it dramatically increases mortality risk and influences patient management strategies [4,5].
Decompensated cirrhosis has traditionally been defined by the development of so-called specific complications associated with portal hypertension and liver dysfunction or “decompensating events”, with the most frequent being: ascites, hepatic encephalopathy, portal hypertension-associated gastrointestinal bleeding, and jaundice; it should be noted that the list of decompensating events is significantly longer and includes infections, especially those associated with cirrhosis-associated immune dysfunction syndrome (CAID) [6], the spectrum of malnutrition–sarcopenia–frailty, lung and cardiac events, etc. [7–10]. Along the same vein, recent research has demonstrated that decompensation does not always evolve uniformly. While some patients experience a sudden onset of severe complications – defined as acute decompensation (AD) – others progress gradually into the decompensated stage, a condition referred to as non-acute decompensation (NAD) [11–13].
Acute decompensation was first characterized in the CANONIC study, which defined AD as the acute (days to few weeks) development of at least one severe complication, such as grade 2–3 ascites, acute hepatic encephalopathy, gastrointestinal bleeding, or any acute bacterial infection [5,14]. These complications can lead to multiple organ/system dysfunction of outright failure termed acute-on-chronic liver failure (ACLF), a subtype of AD characterized by high short-term mortality [15]. Most importantly in the classification, prognostic stratification and personalized management of AD/ACLF is the search for the so-called “trigger of decompensation” in the landmark CANONIC study, where the most frequent triggers were acute infection, clinically significant bleeding, and acute alcohol-associated hepatitis [5]. It should be underscored therefore, that the lists of items in the domains of “triggers” and “events” overlap – and their proper allocation is of paramount importance (e. g., infection can be either a trigger or event or both).
In contrast, in NAD, decompensating events are less severe and evolve more slowly, often insidiously over months without requiring hospitalization, involving a gradual decline in liver function and worsening of portal hypertension [16]. Although NAD is not as immediately life-threatening as AD, it changes the prognosis of patients and poses a significant risk for future AD and worsened patient prognosis [17].
Aim
The aim of this article is to provide a detailed overview of the pathophysiology, clinical manifestations, and classification of AD and NAD. It emphasizes their prognostic implications and identifies key factors contributing to the transition from compensated to decompensated cirrhosis, focusing on the opportunities for early diagnosis, personalized treatment, and complication prevention.
Pathophysiology of acute decompensation
Acute decompensation (AD) of cirrhosis is a complex condition driven by key pathophysiological mechanisms, including portal hypertension, systemic inflammation, bacterial translocation, and organ dysfunction. AD is not merely a clinical phenomenon, but also a consequence of profound systemic changes that significantly influence patient prognosis.
Portal hypertension, defined as an increase in hepatic venous pressure gradient (HVPG) above 5 mmHg, is the most critical factor which usually after achieving the threshold of 10 mmHg (CSPH – called clinically significant portal hypertension) leads to decompensation [3]. Elevated portal pressure triggers the formation of collateral vessels (e. g., esophageal varices), which may rupture and bleed, contributing to the development of ascites, and more or less is directly associated with all the other events [10]. For example, portal hypertension compromises the intestinal barrier, weakening its integrity and promoting the translocation of bacteria and endotoxins into the systemic circulation [11]. This translocation is closely correlated with immune system activation, triggering inflammatory responses and increasing the risk of infections, such as spontaneous bacterial peritonitis (SBP) [10].
Systemic inflammation plays a central role in the onset of AD and its progression to ACLF. A primary driver of inflammation is bacterial translocation, which induces the massive release of pro-inflammatory cytokines into the portal circulation and the liver, including tumor necrosis factor-alpha (TNFα), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) [12,13,18]. These cytokines initiate cascades of processes that primarily damage the liver and then other organs and systems such as the kidneys, brain, circulation, lungs, etc. [4,5].
In addition, patients with cirrhosis frequently suffer from CAID. This condition can have several phenotypes but is most often characterized by impaired neutrophil function, reducing the ability to clear bacteria and thereby exacerbating systemic inflammation and increasing susceptibility to bacterial infections [6,14].
Bacterial infections are present in up to 50% of patients hospitalized with AD and are among the primary triggers of this condition [4,12]. The most common types of infections include: spontaneous bacterial peritonitis (SBP), respiratory tract infections (RTI), urinary tract infections, soft-tissue infections, and spontaneous bacteremia [15]. Infections not only trigger AD, but also substantially elevate the risk of developing ACLF with this risk persisting even after successful infection resolution [15]. These findings underscore the critical importance of effective prevention and early treatment of infections in cirrhotic patients to reduce complications and improve prognosis [19].
Organ failure is a hallmark of ACLF, with the kidneys and circulatory system being most frequently affected [5]. Hepatorenal syndrome-acute kidney injury (HRS-AKI, previously termed HRS-1) is a severe complication characterized by rapid deterioration in renal function. This condition is closely linked to progressive systemic vasodilation and inflammation leading to low mean arterial pressure, significantly impairing perfusion to the kidneys and other vital organs [16,17]. Additionally, cirrhosis is often accompanied by complex hemostatic dysregulation caused by an imbalance between procoagulant and anticoagulant factors. This dysfunction increases the risks of both bleeding and thrombosis, heightening the likelihood of serious complications [4]. Progression from AD to ACLF results from cumulative damage caused by CSPH, inflammation, oxidative stress, and hepatocyte apoptosis [5]. ACLF is defined by the failure of one or more organs, with severity escalating as the number of failing systems increases. Mortality in ACLF is alarmingly high, exceeding 77% at 28 days for patients with grade 3 ACLF [5].
Clinical classification of acute and non-acute decompensation
Cirrhosis decompensation represents a critical clinical and prognostic point that divides the course of the disease into compensated and decompensated stages. Modern classifications differentiate between acute decompensation (AD) and non-acute decompensation (NAD). These two forms are characterized by different dynamics of complication onset, probably distinct pathophysiological mechanisms, and diverse clinical outcomes (Tab. 1) [4,11,18].
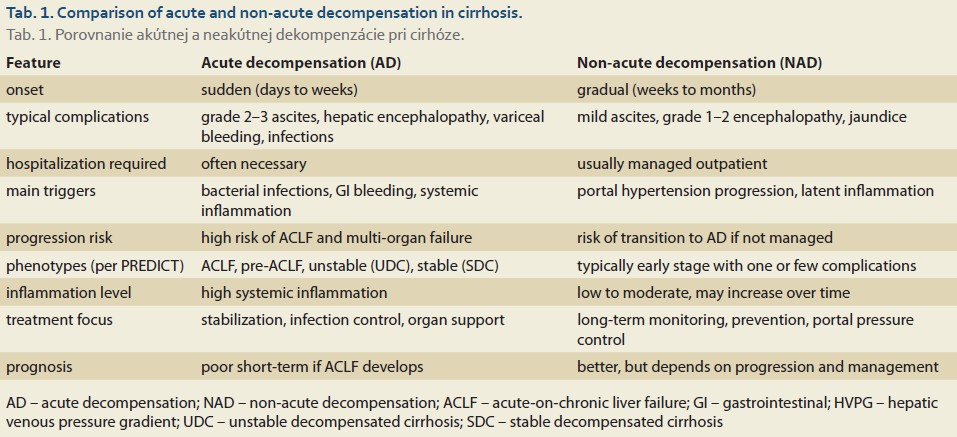
Acute decompensation
AD is defined by the sudden onset of cirrhosis complications, with the speed of their development being a key feature. AD typically arises over days to weeks and includes severe complications (events) such as:
- Grade 2 or 3 ascites: This is characterized by the rapid accumulation of ascitic fluid within two weeks, and is considered a hallmark of AD. It often necessitates hospitalization, diuretic therapy initiation, paracentesis for fluid drainage, and management of frequently associated renal dysfunction [4].
- Hepatic encephalopathy (HE): In AD, HE can manifest as the first or a recurring episode of acute cognitive impairment or altered consciousness in patients with previously normal neuropsychological status. This condition is linked to elevated ammonia levels and other toxic metabolites [11].
- Acute gastrointestinal bleeding: Bleeding from esophageal or gastric varices represents a life-threatening complication, potentially leading to massive blood loss and hemodynamic shock. This event significantly exacerbates systemic inflammation and can accelerate progression to ACLF [5].
- Acute bacterial infections: Infections are not only common triggers of AD, but also major risk factors for progression to ACLF. These infections increase the systemic inflammatory burden and contribute to organ dysfunction [11,12].
The CANONIC study detailed AD as a clinical syndrome involving the combination of these complications. The rapid onset of such events not only defines AD but also significantly impacts short-term patient prognosis, particularly when ACLF develops. AD is therefore considered a dynamic process reflecting acute systemic destabilization [5].
Phenotypes of AD
The PREDICT study identified four distinct phenotypes of AD differentiated by pathophysiology, clinical presentation, and prognosis [13]:
- 1. ACLF (16%): This phenotype is marked by multi-organ failure associated with above-mentioned pathophysiological mechanisms. Patients with ACLF have a poor prognosis with a 28-day mortality rate increasing significantly with the grade from ACLF 1 to ACLF 3. Key contributors to ACLF development include bacterial infections, hemodynamic shock, renal failure, and metabolic disturbances [5,13].
- 2. Pre-ACLF (17%): This transitional phenotype of AD is characterized by pronounced inflammation and a high risk of progression to ACLF during hospitalization. Patients with pre-ACLF often display signs of organ/system instability, such as elevated pro-inflammatory cytokines and organ dysfunction that do not yet meet ACLF criteria as determined by the CLIF-OF calculator. Intensive monitoring and preventive treatment are crucial for these patients [11,13].
- 3. Unstable decompensated cirrhosis (UDC, 22%): Patients with UDC experience recurrent episodes of acute decompensation over a short period of time, often accompanied by inflammation and worsening liver function. This phenotype is typified by chronic clinical deterioration and frequent hospitalizations. Inflammatory processes and complications such as ascites or HE are more pronounced compared to stable forms of decompensation [11].
- 4. Stable decompensated cirrhosis (SDC, 48%): This phenotype encompasses patients who, after an initial episode of decompensation, do not experience further clinical deterioration for at least a year. Although complications such as ascites or mild HE persist, they remain manageable on the outpatient basis. SDC is associated with a better prognosis compared to other AD phenotypes, even though patients remain at risk for future worsening in the absence of proper management [11,13].
This classification of AD/further decompensation spectrum highlights the heterogeneity of AD, enabling a more personalized approach to treatment and risk stratification based on the specific phenotype.
Non-acute decompensation (NAD)
NAD is clinically less dramatic, yet its long-term consequences can be significant. It is characterized by the gradual worsening of liver function and portal hypertension with complications developing more slowly. Key features of NAD include:
- Slow ascites formation: Gradual accumulation of ascitic fluid that does not require urgent hospitalization and is manageable with diuretic therapy is among the most common manifestations of NAD [4].
- Mild hepatic encephalopathy: Encephalopathy of grades 1–2, which can be managed in an outpatient setting [12].
- Progression of jaundice: Occurs in patients with non-cholestatic cirrhosis as a sign of worsening liver function [12].
The PREDICT study demonstrated that NAD is often the first manifestation of cirrhosis decompensation, with approximately 58–72% of patients experiencing only one complication during their first decompensation episode, while 28–42% present with multiple complications [11]. Although NAD may not require immediate hospitalization, it represents a significant risk factor for future AD development. The transition from non-acute decompensation (NAD) to acute decompensation (AD) is a complex process driven by the interplay of various pathophysiological mechanisms. Systemic inflammation plays a key role in this transition, as NAD is frequently accompanied by latent inflammation, which can be exacerbated by bacterial infections or endotoxemia. These conditions lead to the massive release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNFα) and interleukin-6 (IL-6). These cytokines damage the endothelium, increase barrier permeability, and promote systemic failure, including dysfunction of the kidneys, brain, and other organs. Inflammation also triggers widespread systemic vasodilation and increases the risk of sepsis, significantly contributing to disease progression [5]. Another critical factor is the increase in portal pressure, a hallmark of cirrhosis progression. Chronic fluid accumulation in the abdominal cavity facilitates complications such as spontaneous bacterial peritonitis (SBP). SBP serves as a major trigger for sudden clinical deterioration and can lead to multi-organ failure if not promptly diagnosed and treated [4]. In NAD, homeostatic mechanisms are weakened, greatly impairing the body’s ability to adapt to circulatory changes. The kidneys often lose their capacity to respond to hemodynamic shifts, resulting in hepatorenal syndrome with acute kidney injury (HRS-AKI). This condition is characterized by severe vasoconstriction and inadequate renal perfusion, accelerating the transition to AD. Furthermore, cardiovascular compensatory failure contributes to insufficient perfusion of vital organs, worsening the overall condition [12]. Progression from NAD to AD can also be influenced by CAID. This disorder reduces the body’s ability to eliminate bacteria, facilitating bacterial translocation and subsequent infections. Diminished immune response undermines the capacity to manage inflammation, potentially becoming a decisive factor in disease progression [14]. These mechanisms together create a dynamic, nonlinear process that may be triggered by various stressors. Identifying and controlling these factors is crucial for preventing severe complications and improving outcomes for patients with cirrhosis. The transition from NAD to AD has been traditionally seen as clinically unpredictable. However, the PREDICT study identified several risk factors indicating a higher likelihood of this progression:
- 1. Presence of portal hypertension: Patients with HVPG > 10 mmHg have a significantly higher risk of acute deterioration [4].
- 2. History of mild complications: Patients with NAD who have previously experienced ascites or encephalopathy are more susceptible to a sudden onset of AD [11].
- 3. Bacterial infections: Spontaneous bacterial peritonitis and other infections increase the likelihood of systemic destabilization and subsequent transition to AD [12].
On the other hand, adequately managed NAD through outpatient treatment can delay or even prevent progression to AD (18). This includes thorough monitoring of portal pressure, timely diagnosis of infections, and early management of mild complications.
Prognosis of patients with NAD and AD
The prognosis of NAD and AD differs significantly due to the varying severity of clinical manifestations and systemic dysfunctions. While NAD is usually associated with a gradual decline in liver function, AD represents an acute clinical crisis with potentially high short-term mortality [5,11].
Elevated portal pressure (HVPG > 16 mmHg) remains one of the most significant prognostic markers, negatively affecting survival in patients with both NAD and AD [4,12]. Bacterial infections, such as spontaneous bacterial peritonitis or pneumonia, significantly increase the risk of further complications and mortality, even when clinically managed [11]. Similarly, kidney failure in the context of HRS-AKI represents a critical factor that severely worsens clinical outcomes and prognosis in patients with AD [5]. These factors collectively underscore the need for early diagnosis, prevention, and targeted treatment to minimize complications and improve the patients‘ quality of life.
Management and treatment of acute and non-acute decompensation
Treatment of decompensated cirrhosis is complex and requires a multidisciplinary approach that considers the specific characteristics of acute decompensation (AD) and non-acute decompensation (NAD). Management of these two conditions differs significantly, as AD represents an urgent clinical situation, while NAD requires a long-term strategy to prevent disease progression.
Treatment of AD
Management of AD involves multiple steps to stabilize the patient and address complications:
- 1. Patient stabilization upon admission: Patients with AD should be hospitalized in a setting with the capacity for intensive monitoring and management of complications. Care includes assessing circulatory and respiratory status, administering oxygen for respiratory insufficiency, and securing venous access [4]. For hemodynamic failure, immediate infusion therapy with crystalloids or albumin is recommended to restore intravascular volume and prevent organ failure [5].
- 2. Management of ascites: Diagnostic and therapeutic paracentesis is essential for massive ascites, with fluid loss exceeding 5 liters requiring albumin replacement to prevent circulatory collapse [5,11]. Long-term control is achieved using a combination of spironolactone and furosemide with regular monitoring of renal function and electrolytes [4]. In recalcitrant cases (resistant/refractory ascites), besides the first-line approach with the outpatient repeated large-volume paracentesis with albumin replacement, second-line therapies should be considered – such as transjugular intrahepatic portosystemic shunt (TIPS) and peritoneal indwelling-catheter based therapies such as alpha-pump or liver transplantation.
- 3. Treatment of hepatic encephalopathy (HE): Dietary and lifestyle intervention combined with lactulose is considered the first-line therapy of HE; lactulose p. o. reduces intestinal ammonia absorption, and is the cornerstone of HE management [4]. If insufficient, rifaximin can be added as the second-line therapy influencing ammonia-producing bacteria [11].
- 4. Control of variceal bleeding: Endoscopic variceal ligation (EVL) is the standard treatment for acute bleeding. Acutely, pharmacological therapy with terlipressin reduces portal pressure [10], while beta-blockers such as carvedilol are effective in primary and secondary prevention [4].
- 5. Management of bacterial infections: Empirical antibiotic therapy should be managed according to the diagnosis (SBP, RTI, etc.), and according to the monitored local prevalence of resistant species. Long-term primary and secondary prophylaxis of SBP with norfloxacin is recommended in properly selected patients, but this remains the point of discussion because of bacterial resistance issues [4,11].
- 6. Renal support: Hepatorenal syndrome (HRS) is treated with a combination of management of triggers/risk factors such as drugs and infections, together with the combination of terlipressin and albumin. Dialysis may be required if pharmacotherapy fails and may be combined with liver-kidney transplantation after a certain period of time as needed [5].
- 7. Anti-inflammatory and immunomodulatory therapy: As the importance of the inflammatory domain in pathogenesis of AD/ACLF increases, it may be seen as an evolving potential therapeutic target. Currently, albumin provides immunomodulatory effects in addition to hemodynamic benefits, reducing systemic inflammation. Long-term albumin administration can improve patient outcomes [14].
Treatment of NAD
Management of NAD focuses on preventing complications and achieving long- -term clinical improvement. The most important first step in any prevention in this context is to establish and treat the underlying etiology of ACLD – e. g., antiviral therapy of replicating infections in hepatitis B and hepatitis C, integrated addictology care in patients with ALD and active alcohol consumption, etc. Other general measures include:
- 1. Control of portal hypertension: Non--selective beta-blockers are the cornerstone of therapy, reducing the risk of variceal bleeding and lowering portal pressure. The goal is to reduce heart rate by 25% from baseline for non-selective beta-blockers, but it is more tricky to lead therapy with administered combined-mechanism carvedilol [4,10].
- 2. Diuretic therapy for mild ascites: In NAD, diuretics are used at lower doses than in AD to avoid renal dysfunction. Regular monitoring of electrolytes and renal function is critical [12].
- 3. Nutritional support: A diet rich in proteins and sufficient in calories is essential together with supervised intervals between meals shorter than 4 hours [20,21]. Supplements such as branched-chain amino acids (BCAAs) can help prevent sarcopenia and improve overall nutritional status [4,22].
- 4. Long-term monitoring: Regular follow-up includes monitoring liver function (bilirubin, INR levels), ascites, and signs of portal hypertension. The objective is to detect the transition to AD early on [11].
Recommendations for future research and perspectives in cirrhosis management
Decompensated cirrhosis is a complex clinical problem that requires innovative approaches to improve diagnosis and treatment. Acute decompensation (AD), characterized by a sudden deterioration in clinical status, demands rapid and intensive care, whereas non-acute decompensation (NAD) progresses more slowly and emphasizes a long-term strategy for preventing complications.
One of the primary areas of research is inflammation and immunomodulation, as systemic inflammation plays a key role in the pathogenesis of AD. Immunomodulatory approaches, such as selective modulators of inflammatory pathways and expanded use of albumin, can significantly improve patient prognosis by reducing inflammation and minimizing organ damage. These strategies are particularly promising for preventing progression from acute-on-chronic liver failure (ACLF) [14].
Another promising area is the development of non-invasive biomarkers to better predict the transition from NAD to AD [13,18]. Molecules such as microbial DNA, inflammatory cytokines, or metabolites can enhance patient risk stratification and support more precise therapeutic decision-making. Such diagnostic tools could also reduce the need for invasive interventions [15,16].
A key phenomenon in cirrhosis management is recompensation, which refers to the improvement of liver function and the return of a patient from a deteriorated clinical stage to a more stable condition. This phenomenon is often observed following antiviral therapy for hepatitis C or interventions such as transjugular intrahepatic portosystemic shunt (TIPS) or beta-blockers to reduce portal pressure. While recompensation does not equate to a complete cure of cirrhosis, it enables significant improvements in quality of life and patient survival. Research in this area should focus on identifying mechanisms that promote liver regeneration and developing therapies to optimize this process [17,22].
Future perspectives
Decompensated cirrhosis remains a multidimensional challenge requiring a combination of advanced technologies and personalized medicine. While current therapeutic approaches can improve short-term outcomes, long-term patient survival will depend on effective prevention of complications and support for liver regeneration. The integration of immunomodulatory therapies, biomarker-based diagnostics, and interventional techniques such as TIPS offers a pathway to reduce disease burden and extend patient survival. With increasing emphasis on personalized management and research into recompensation mechanisms, significant advancements in the care and clinical outcomes of patients with cirrhosis can be anticipated.
Conclusion
Decompensation in cirrhosis marks a turning point in disease progression and prognosis. Differentiating between acute and non-acute forms allows for more targeted management. Early identification, control of inflammation and portal hypertension, and prevention of complications are key to improving outcomes. Focus on personalized treatment and the potential for recompensation offers hope for better long-term care.
ORCID authors
D. Žilinčanová 0009-0004-7100-0654,
S. Adamcová Selčanová 0000-0001-8181-1937,
D. Havaj 0000-0001-5979-8326,
K. Šulejová 0009-0005-4149-3694,
T. Takáč 0009-0007-5785-7573,
Ľ. Skladaný 0000-0001-5171-3623.
Submitted/Doručené: 25. 3. 2025
Accepted/Prijaté: 2. 4. 2025
Corresponding author
Daniela Žilinčanová, MD
2nd Department of Internal Medicine
Div. HEGITO (Hepatology, Gastroenterology, and Transplantation Department)
Slovak Medical University Faculty of Medicine, Roosevelt University Hospital
Nám. L. Svobodu 1
975 17 Banská Bystrica
daniela.zilincanova@gmail.com
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis. J Hepatol 2006; 44(2): 217–231. doi: 10.1016/j.jhep.2005.10.013.
2. Asrani SK, Devarbhavi H, Eaton J et al. Burden of liver diseases in the world. J Hepatol 2019; 70(1): 151–171. doi: 10.1016/j.jhep.2018.09.014.
3. Bosch J, Abraldes JG, Berzigotti A et al. Portal hypertension and gastrointestinal bleeding. J Hepatol 2008; 48(Suppl 1): S68–S92. doi: 10.1016/j.jhep.2008.01.021.
4. European Association for the Study of the Liver. EASL clinical practice guidelines for the management of decompensated cirrhosis. J Hepatol 2018; 69(2): 406–460. doi: 10.1016/ j.jhep.2018.03.024.
5. Moreau R, Jalan R, Gines P et al. Acute-on-chronic liver failure is a distinct syndrome. Gastroenterology 2013; 144(7): 1426–1437. doi: 10.1053/j.gastro.2013.02.042.
6. Havaj DJ, Skladaný L. Cirrhosis-associated immune dysfunction (CAID) – causes, phenotypes and consequences. Gastroenterol Hepatol 2022; 76(2): 101–110. doi: 10.48095/ccgh2022101.
7. Vrbová P, Koller T. Význam sarkopénie a krehkosti v manažmente cirhózy. Gastroenterol Hepatol 2021; 75(2): 102–109. doi: 10.48095/ccgh2021102.
8. Sekereš M. Rehabilitácia pri cirhóze pečene. Rehabilitácia 2023; 60 (3): 184–196. doi: 10.61983/lcrh.v60i3.5
9. Wang S, Whitlock R, Xu C, Taneja S et al. Frailty is associated with increased risk of cirrhosis disease progression and death. Hepatology 2022; 75 (3): 600–609. doi: 10.1002/hep.32157.
10. Garcia-Tsao G, Sanyal AJ, Grace ND et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46(3): 922–938. doi: 10.1002/hep.21907.
11. Trebicka J, Fernandez J, Papp M et al. The PREDICT study: a multicenter observational study on the predictive value of pre-acute-on-chronic liver failure syndrome in cirrhosis. J Hepatol 2020; 73(4): 842–854.
12. Tonon M, Piano S, Romano A et al. Patterns of decompensation in cirrhosis: insights from the PREDICT study. J Hepatol 2024; 80(4): 603–609.
13. D‘Amico G, Bernardi M, Angeli P. Towards a new definition of decompensated cirrhosis. J Hepatol 2022; 76(1): 202–207. doi: 10.1016/j.jhep.2021.06.018.
14. Jalan R, Mookerjee RP, Reiberger T et al. Albumin in decompensated cirrhosis: from pathophysiology to clinical practice. J Hepatol 2021; 75(5): 1232–1242.
15. Bernardi M, Moreau R, Angeli P et al. Systemic inflammation in decompensated cirrhosis: clinical implications and management. J Hepatol 2015; 64(1): 40–50.
16. Bajaj JS, Heuman DM, Sanyal AJ et al. Gut microbiome and its interaction with the host in cirrhosis. Nat Rev Gastroenterol Hepatol 2019; 16(2): 108–120.
17. Reiberger T, Mandorfer M. Beta-blockers and cirrhosis: shifting paradigms. Gut 2021; 70(10): 2155–2165.
18. Tonon M, D’Ambrosio R, Calvino V et al. A new clinical and prognostic characterization of the patterns of decompensation of cirrhosis. J Hepatol 2024; 80(3): 603–609. doi: 10.1016/ j.jhep.2023.12.005.
19. Bystrianska N, Skladaný Ľ, Adamcova Selcanova S et al. Risk factors for hepatic encephalopathy in patients with liver cirrhosis. Gastroenterol Hepatol 2020; 74(2): 111–115. doi: 10.14735/amgh2020111.
20. Plauth M, Bernal W, Dasarathy S et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019; 38(2): 485–521. doi: 10.1016/ j.clnu.2018.12.022.
21. Merli M, Giusto M, Gentili F, Novelli G et al. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int 2010; 30(2): 208–214. doi: 10.1111/j.1478-3231.2009.02135.x.
22. Villanueva C, Graupera I, Aracil C et al. A randomized controlled trial of long-term effects of TIPS in patients with cirrhosis. Hepatology 2014; 60(1): 289–300.